pH: Acid vs Base
The pH scale and how it indicates acidity or basicity.
Acids & Bases
classifying substances as acids or bases
H2O → H+ + OH-
The chemical equation above displays the dissociation of water molecules into hydrogen (H+) and hydroxide (OH–) ions. This reaction is essential in examining the differences between acids and bases.
Acids
An acid is a substance that increases the concentration of H+ ions in solution. The acid dissociates into H+ and its negative counterpart, known as the conjugate base. For example, hydrochloric acid (HCl) dissociates to produce H+ and Cl–. The written reaction is as follows: HCl → H+ + Cl– .
Bases
A base is a substance that reduces the concentration of H+ ions in solution. This occurs when a negatively charged ion combines with H+ to form a neutrally charged molecule. Commonly H+ ions can combine with hydroxide ions (OH–) to form water as shown in the equation above.
pH Scale
indicating acidity and basicity
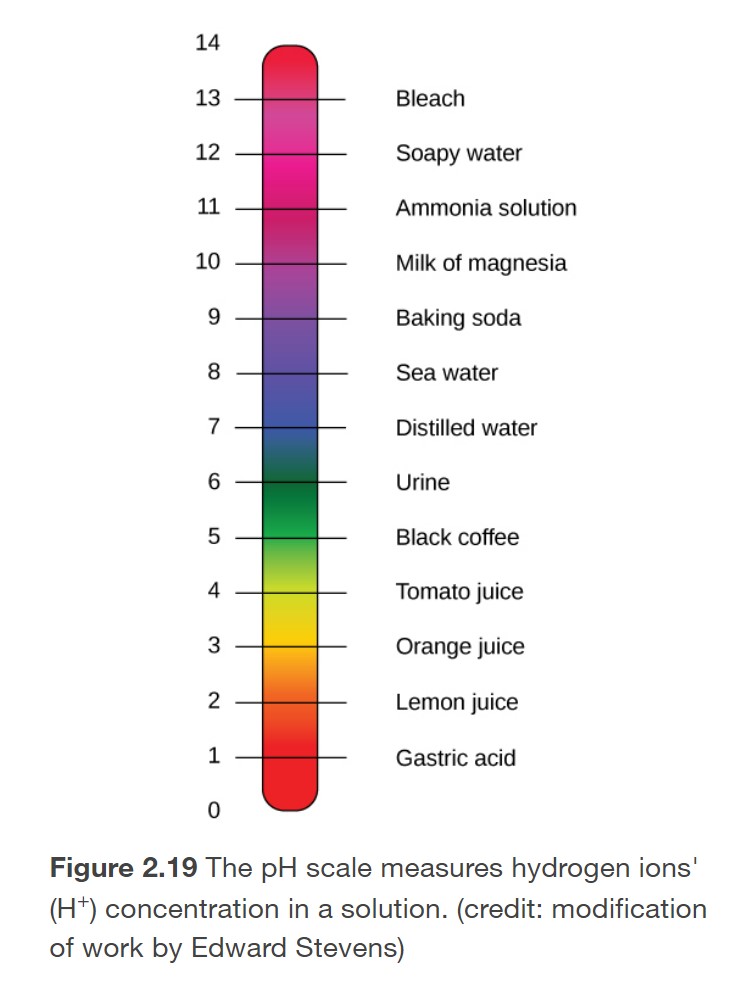
The pH of a substance indicates its acidity or basicity. pH can be determined based on the concentration of H+.
pH = -log [H+]
Neutral substances have a pH of 7. pH values below 7 indicate acidity, while pH values greater than 7 indicate basicity. As shown in the image to the left, many foods and drinks are acidic. For example, lemon juice and orange juice are acidic as they both contain citric acid, which dissociates producing
