Food Colorings & Additives
Natural and artificial food additives.
Food Colorings
Food colorings are used to make foods look more appealing as well as to indicate flavorings or ingredients. Think about candies, like Skittles! Each Skittle is colored to correspond to each flavor. Would you eat Skittles if they were all white?! Probably not!
How do food colorings work?
Effective food colorings must be water-soluble in order to ensure a consistent color. Therefore, most food colorings are composed of ionic solids, which dissociate into positive & negative ions. These ions attract to the slightly charged portions of polar water molecules and, therefore, remain in solution.
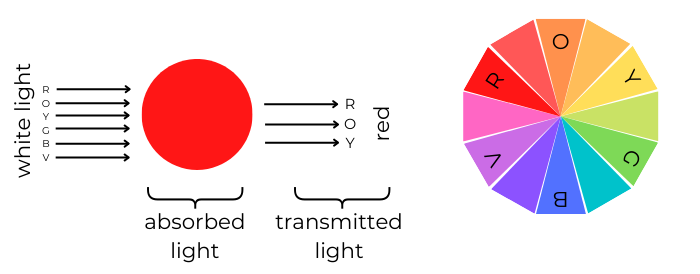
Food colorings must remain colored when dissolved. In order for this to occur, the colorings must readily absorb some wavelengths of visible light (400-700 nm) and transmit other wavelengths of visible light. The diagram below shows how food colorings absorb and transmit light. First, white light is exposed to the colored substance; this white light contains a range of wavelengths that includes all visible light from red to violet. As stated earlier, the colored substance absorbs some wavelengths of light. In the case below (of a red substance), green, blue, and violet light are absorbed. The absorbed light falls within the wavelength range of complementary colors (those directly opposite on the color wheel) of the substance. As seen within the color wheel, the complementary color of red is green, so a range of wavelengths, including that of green light, are absorbed. The wavelengths not absorbed are transmitted. This transmitted light is what we see. Below, we see that red, orange, and yellow light are transmitted. This is why the substance appears red!
Natural Colorings
Carotenoids
red, orange, or yellow
Carotenoids are soluble in fat so they are typically used to color dairy products like cheese.
Chlorophyll
green
Chlorophyll is a natural green pigment found in plants. It plays an important role in capturing light energy during photosynthesis.
Anthocyanin
purple or blue
Anthocyanins are soluble in water so they are used to color water-based products like soft drinks.
Turmeric
orange/yellow
Turmeric is a spice obtained from an Indian plant. This spice is used to give mustard its deep yellow color.
Artificial Colorings
Artificial food colorings are currently derived from petroleum. However, the dyes are extensively tested to ensure that no petroleum remains after production.
WHY ARTIFICIAL?
- cheaper to produce
- longer-lasting
- wider range of colors
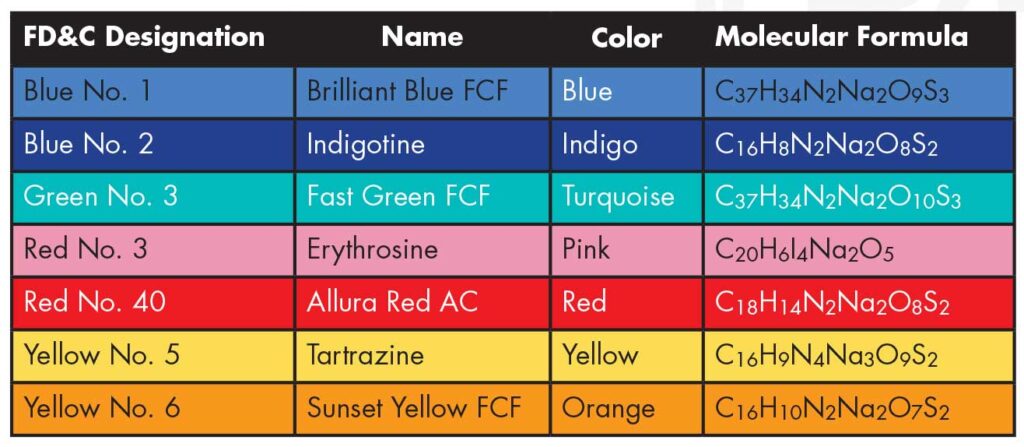
The dyes approved by the U.S. Food & Drug Administration are listed above.
Food Flavorings
What is flavor?
The flavor of food is reliant of taste, smell, texture, temperature, and preparation. There are five basic tastes: sweet, bitter, sour, salty, and umami. Umami is a taste associated with rich, savory foods like meats and broths. While we don’t typically associate smell with flavor, smell is plays a significant roll in body’s ability to recognize flavor. As you chew food, aromas travel to sensory cells in your nose. These aromas add to the taste of the food. Think about the times you’ve been sick. Did foods taste the same as they normally did? Probably not! If your nose is congested, aromas from food can’t travel to the nose. Without smell, food doesn’t have the same flavor!
Many of the foods we eat daily contain food flavorings derived either naturally or artificially. For example, some juices contain many chemicals that combine to create a flavor very similar to the real fruit juice, while other juices are made from concentrates extracted directly from fruit.
Natural
The natural flavors of fruits, vegetables, and other foods are composed of hundreds of chemicals. Chemists study these chemicals in order to create variations that can be produced synthetically.
Artificial
Artificial flavors are derived from chemicals present in plants and animals. Some chemicals are used in their natural state, while others are transformed. The goal of artificial flavorings is to synthetically produce a flavoring that is similar to the representative food, which also being more efficient to produce. Instead of requiring hundreds of chemicals to create a flavoring, a few of the most abundant can be used.
Esters
Esters are chemical compounds often used to generate an aroma. They are commonly used to create scents for artificially derived scents or flavors or products like soap, juice, and gum. Ester compounds form when an alcohol reacts with a carboxylic acid.
- Carboxylic acids are weak organic acids that have the general formula R-COOH. The “R” is any hydrocarbon group. “COOH” is a carboxyl group. The hydrogen bonded to the oxygen is the only acidic proton present within the acid.
- An alcohol is an organic compound that has the general formula R-OH. A hydrocarbon group is bonded to a hydroxyl group.
Different combinations of carboxylic acids and alcohols can be used to produce different flavors. For example, ethanol and butanoic acid combine to produce the ester Ethyl butanoate, which has a pineapple scent.
Nomenclature
All of these compounds contain carbon atoms, which classifies them as organic compounds. Their prefixes are determined based on the number of carbon atoms present in their hydrocarbon backbone. Hydrocarbons are named for the longest chain of carbon atoms followed by the suffix -ane. For example, a hydrocarbon containing only one carbon is named methane.
Alkanes are saturated hydrocarbons that contain only single covalent bonds between carbon and hydrogen atoms. They are named following the same naming system as above. Alkyl groups are substituents that contain one less hydrogen than the corresponding alkane. These are name by changing the -ane suffix of the corresponding alkane to –yl. For example, an alkyl group with one carbon and three hydrogens is considered a methyl group. These groups can be attached to an alkane, creating a larger and more complex hydrocarbon.
Carboxylic Acids
Carboxylic acids contain a carboxyl group (-COOH) as well as a hydrocarbon group (R) The second oxygen atom in these acids is bonded to a hydrogen atom.
The nomenclature of carboxylic acids is dependent on the hydrocarbon structure as discussed above. The -e at the end of the alkane name is dropped and -oic acid is added.
For example:
This molecule contains a 2 carbon chain. Therefore, its hydrocarbon base is ethane but, because it contains a carboxyl group, it is a carboxylic acid. Therefore, the name ethane is transformed into ethanoic acid.
Alcohols
Alcohols are derivatives of hydrocarbons in which a hydroxyl group (-OH) has replaced a hydrogen atom. Alcohols are covalent molecules.
Once again, the nomenclature of alcohols is based on its hydrocarbon structure. First, identify the hydrocarbon base. Then, change the -e suffix to -ol. Finally, identify the number carbon in which the hydroxyl group is attached. This number is placed before the name.
For example:
This molecule contains 5 carbons, which indicates that the hydrocarbon base is pentane. Then, the -e is changed to -ol, changing pentane to pentanol. Finally, the hydroxyl group is bonded to the second carbon. Therefore, this alcohol is named 2-pentanol.
Esters
Esters contain a carboxyl group as well as two hydrocarbon groups (R). The second oxygen bonds to another carbon atom.
When naming esters, the name of the corresponding carboxylic acid is transformed by changing the -ic ending to -ate. The name of the attached alkyl group is put before the name of the ester.
For example:
This molecule contains a 2 carbon chain (ethane), a carboxyl group, and an alkyl group. This identifies it as an ester. The alkyl group contains one carbon, which makes it a methyl group. Therefore, this ester is named methyl ethanoate.
For more detailed information on organic nomenclature visit Chapter 20 in Chemistry 2e!
